COVID-19
Part IV: Clerical Errors Affect Real People!

Medical clerical staff are significant workers in the health centres.
Not only do they support the doctors and nurses in their roles, but they also ensure accurate results which turn into statistical analysis for future treatment recommendations.
But consider the case of my mother, who was allegedly diagnosed with Covid 19 at a seniors’ home and consequently spent two weeks in isolation (quarantine) as per government policy.
Nearly two weeks later, a note was added to her file of which the content follows:
November 27, 2020
Dear Resident/Family Member
I am writing to you to confirm that we have had no other residents at …. test
positive for COVID-19. With that being said, we have taken many residents off isolation today
due to a clerical error from AHS that resulted in a false positive reporting.
The director of the facility ends the letter off with an interesting paragraph:
Please also know that the best defense against the spread of this virus are actions that are well
within each of our control: stay home as much as possible, practice physical distancing (2
metres)/ wash your hands regularly/ use good cough etiquette and avoid touching your mouth.
Without playing the victim card, what is the consequence of this clerical error to the individual who made the error?
For my mother, she lost 2 weeks of her life isolated in her apartment with a hazmat suit, masks and gloves in front of her unit. She could not receive visitors and was not able to see her family.

Like any senior, student, teacher or worker who may have received a false positive, they are not faceless or nameless. Errors have real life consequences.
This marks the 5th time of isolation in the retirement home. Of these 5 times, ALL were due to policy i.e. 2-week isolation for a negative test or returning from a trip to visit family. While initially based on a positive indicator, this last circumstance was triggered by a hallway disinfection during which she had coughing symptoms and a test was administered. It turns out the particular disinfectant used by the home may trigger a coughing reaction.
However, the test was conducted and the positive was overturned. Mea Culpa.
I have to wonder what the clerical staff who erred received for their gaffe? The note is not clear as to if the clerical error was on the part of the technician or the individual entering the results. Either is unacceptable-technical or clerical side. Or the alternate questions, how many other people had their lives turned upside down due to the error? We also have to wonder how many people were contact traced and as well had to isolate?
We can probably estimate that for each false positive, 5 people were requested to be tested and if the test was incorrect OR the clerical staff erred there could be as many as 50 false results that day.
Province wide, what was the impact on the daily fright report? If again, 50 people were false, our daily numbers would fall. Perhaps more results were incorrect? We do not know, but we do know that peoples’ lives are not to be tampered with and such activities should not be merely accepted.
Extending the argument system-wide, it is these types of errors that continue widespread criticism of our response to the virus. Clerical errors can cause elevated numbers and create more panic (and thereby justify more extreme measures) just as inaccurate or no reporting of other diagnosis such as the influenza and related deaths, suicides, automobile accident fatalities, drug overdoses due to depression and potential prescription related deaths (#3 in the US).
It is well know by anyone who has undergone physiotherapy for shoulder or leg injuries that if your left arm is injured that you will over compensate on the right side. Therefore as one limb heals, the other can also be injured leading to another cycle of physio. The same principle should apply to our health system.
While Covid 19 is a ‘real’ virus with real world threat, it must be considered as part of a larger pie to give world citizens a balanced view of our national health threats else our go to strategy for health management is crisis instead of calm and long term nutritional and holistic approaches.
Clerical errors not withstanding, errors must be publicly acknowledged and corrected. Incorrect positive tests (cases) must be modified and appropriate actions taken to ensure honesty in health reporting. The citizens of our cities, provinces and countries deserve truth from our health providers and ministries. Responsibility and accountability MUST be part of a responsible and responsive health system.
To take a quote out of context, “One small misstep for man, one large misstep for mankind.”
COVID-19
Court compels RCMP and TD Bank to hand over records related to freezing of peaceful protestor’s bank accounts

The Justice Centre for Constitutional Freedoms announces that a judge of the Ontario Court of Justice has ordered the RCMP and TD Bank to produce records relating to the freezing of Mr. Evan Blackman’s bank accounts during the 2022 Freedom Convoy protest.
Mr. Blackman was arrested in downtown Ottawa on February 18, 2022, during the federal government’s unprecedented use of the Emergencies Act. He was charged with mischief and obstruction, but he was acquitted of these charges at trial in October 2023.
However, the Crown appealed Mr. Blackman’s acquittal in 2024, and a new trial is scheduled to begin on August 14, 2025.
Mr. Blackman is seeking the records concerning the freezing of his bank accounts to support an application under the Charter at his upcoming retrial.
His lawyers plan to argue that the freezing of his bank accounts was a serious violation of his rights, and are asking the court to stay the case accordingly.
“The freezing of Mr. Blackman’s bank accounts was an extreme overreach on the part of the police and the federal government,” says constitutional lawyer Chris Fleury.
“These records will hopefully reveal exactly how and why Mr. Blackman’s accounts were frozen,” he says.
Mr. Blackman agreed, saying, “I’m delighted that we will finally get records that may reveal why my bank accounts were frozen.”
This ruling marks a significant step in what is believed to be the first criminal case in Canada involving a proposed Charter application based on the freezing of personal bank accounts under the Emergencies Act.
Alberta
COVID mandates protester in Canada released on bail after over 2 years in jail

Chris Carbert (right) and Anthony Olienick, two of the Coutts Four were jailed for over two years for mischief and unlawful possession of a firearm for a dangerous purpose.
From LifeSiteNews
The “Coutts Four” were painted as dangerous terrorists and their arrest was used as justification for the invocation of the Emergencies Act by the Trudeau government, which allowed it to use draconian measures to end both the Coutts blockade and the much larger Freedom Convoy
COVID protestor Chris Carbert has been granted bail pending his appeal after spending over two years in prison.
On June 30, Alberta Court of Appeal Justice Jo-Anne Strekaf ordered the release of Chris Carbert pending his appeal of charges of mischief and weapons offenses stemming from the Coutts border blockade, which protested COVID mandates in 2022.
“[Carbert] has demonstrated that there is no substantial likelihood that he will commit a criminal offence or interfere with the administration of justice if released from detention pending the hearing of his appeals,” Strekaf ruled.
“If the applicant and the Crown are able to agree upon a release plan and draft order to propose to the court, that is to be submitted by July 14,” she continued.
Carbert’s appeal is expected to be heard in September. So far, Carbert has spent over two years in prison, when he was charged with conspiracy to commit murder during the protest in Coutts, which ran parallel to but was not officially affiliated with the Freedom Convoy taking place in Ottawa.
Later, he was acquitted of the conspiracy to commit murder charge but still found guilty of the lesser charges of unlawful possession of a firearm for a dangerous purpose and mischief over $5,000.
In September 2024, Chris Carbert was sentenced to six and a half years for his role in the protest. However, he is not expected to serve his full sentence, as he was issued four years of credit for time already served. Carbert is also prohibited from owning firearms for life and required to provide a DNA sample.
Carbert was arrested alongside Anthony Olienick, Christopher Lysak and Jerry Morin, with the latter two pleading guilty to lesser charges to avoid trial. At the time, the “Coutts Four” were painted as dangerous terrorists and their arrest was used as justification for the invocation of the Emergencies Act by the Trudeau government, which allowed it to use draconian measures to end both the Coutts blockade and the much larger Freedom Convoy occurring thousands of kilometers away in Ottawa.
Under the Emergency Act (EA), the Liberal government froze the bank accounts of Canadians who donated to the Freedom Convoy. Trudeau revoked the EA on February 23 after the protesters had been cleared out. At the time, seven of Canada’s 10 provinces opposed Trudeau’s use of the EA.
Since then, Federal Court Justice Richard Mosley ruled that Trudeau was “not justified” in invoking the Emergencies Act, a decision that the federal government is appealing.
-

 Alberta10 hours ago
Alberta10 hours agoAlberta Independence Seekers Take First Step: Citizen Initiative Application Approved, Notice of Initiative Petition Issued
-
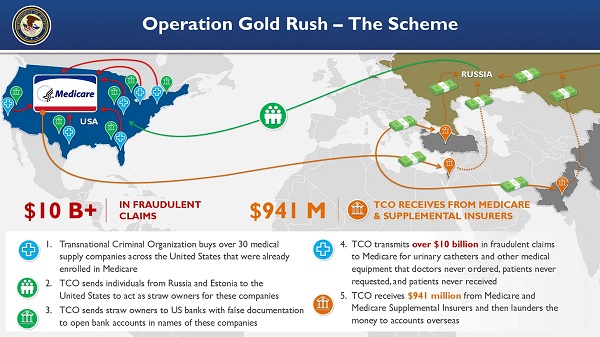
 Crime9 hours ago
Crime9 hours agoNational Health Care Fraud Takedown Results in 324 Defendants Charged in Connection with Over $14.6 Billion in Alleged Fraud
-

 Health8 hours ago
Health8 hours agoRFK Jr. Unloads Disturbing Vaccine Secrets on Tucker—And Surprises Everyone on Trump
-

 Bruce Dowbiggin11 hours ago
Bruce Dowbiggin11 hours agoThe Game That Let Canadians Forgive The Liberals — Again
-

 Alberta1 day ago
Alberta1 day agoCOVID mandates protester in Canada released on bail after over 2 years in jail
-

 Crime2 days ago
Crime2 days agoProject Sleeping Giant: Inside the Chinese Mercantile Machine Linking Beijing’s Underground Banks and the Sinaloa Cartel
-

 Alberta2 days ago
Alberta2 days agoAlberta uncorks new rules for liquor and cannabis
-

 Business1 day ago
Business1 day agoCanada’s loyalty to globalism is bleeding our economy dry