COVID-19
Freedom Convoy leaders’ trial concludes for 2023 but will resume in 2024

From LifeSiteNews
The trial, which began on September 5, 2023, was supposed to have lasted just over two weeks.
The trial against Freedom Convoy leaders Tamara Lich and Chris Barber has concluded for 2023 but will be back again early next year.
Last Thursday, day 33, was the last day of the trial for 2023. The court is expected to resume on January 4, 2024.
During day 33 of the trial, the Democracy Fund (TDF), which is crowdfunding Lich’s legal costs, noted that the court will resume in 2024 with a “voir dire,” or trial within a trial, to be “held over how comments made by the judge presiding over the Ottawa injunction order of February 2022 should be treated.”
“In the days following, there should be a decision on the defense motion to dismiss the Carter application,” noted TDF.
The trial, which began on September 5, 2023, was supposed to have lasted only a few weeks.
Last year, lawyers for both sides agreed that 16 days would be a reasonable amount of time to have a fair case. The Crown however took a long time in going through its witness list, which slowed the pace of the trial to a crawl.
On Day 33 of the trial, legal counsel for Barber reiterated to the court that it’s not a “crime” that the leaders did not tell people to leave Ottawa, as the Crown claims it was.
Thus far, per TDF, the Crown has asserted “that the absence of violence or peaceful nature of the protest didn’t make it lawful, emphasizing that the onus was on the Crown to prove the protest’s unlawfulness.”
The Crown in court has been holding steadfast to the notion in trying to prove that Lich and Barber had somehow influenced the protesters’ actions through their words as part of a co-conspiracy. This claim has been rejected by the defense as weak.
To back the claims up, the Crown has been hoping to use what is called a “Carter application” to help them make their case. The Crown’s so-called “Carter Application” asks that the judge to consider “Barber’s statements and actions to establish the guilt of Lich, and vice versa,” TDF stated.
TDF has said that a Carter application is very “complicated” and requires that the Crown prove “beyond a reasonable doubt” that there was a “conspiracy or plan in place and that Lich was a party to it based on direct evidence,” and as such, the defense is asking the judge to dismiss the application.
The reality is that Lich and Barber worked with police on many occasions so that the protests were within the law.
On day 32 of Lich Barber’s trial, the defense counsel for the leaders exposed gaps in the Crown’s main argument that the protests were unlawful even though there was no violence during the demonstrations.
Lich and Barber are facing multiple charges from the 2022 protests, including mischief, counseling mischief, counseling intimidation and obstructing police for taking part in and organizing the anti-mandate Freedom Convoy. As reported by LifeSiteNews at the time, despite the non-violent nature of the protest and the charges, Lich was jailed for weeks before she was granted bail.
In early 2022, the Freedom Convoy saw thousands of Canadians from coast-to-coast come to Ottawa to demand an end to COVID mandates in all forms. Despite the peaceful nature of the protest, Prime Minister Justin Trudeau’s government enacted the Emergencies Act on February 14.
During the clear-out of protesters after the EA was put in place, one protester, an elderly lady, was trampled by a police horse, and one conservative female reporter was beaten by police and shot with a tear gas canister.
Lich and Barber’s trial has thus far taken more time than originally planned. LifeSiteNews has been covering the trial extensive.
COVID-19
FDA requires new warning on mRNA COVID shots due to heart damage in young men
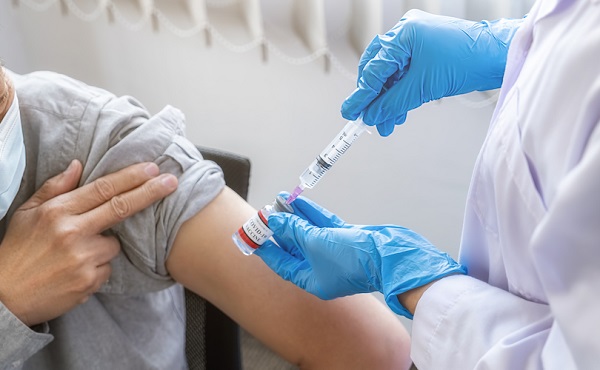
From LifeSiteNews
Pfizer and Moderna’s mRNA COVID shots must now include warnings that they cause ‘extremely high risk’ of heart inflammation and irreversible damage in males up to age 24.
The Trump administration’s Food and Drug Administration (FDA) announced it will now require updated safety warnings on mRNA COVID-19 shots to include the “extremely high risk” of myocarditis/pericarditis and the likelihood of long-term, irreversible heart damage for teen boys and young men up to age 24.
The required safety updates apply to Comirnaty, the mRNA COVID shot manufactured by Pfizer Inc., and Spikevax, the mRNA COVID shot manufactured ModernaTX, Inc.
According to a press release, the FDA now requires each of those manufacturers to update the warning about the risks of myocarditis and pericarditis to include information about:
- the estimated unadjusted incidence of myocarditis and/or pericarditis following administration of the 2023-2024 Formula of mRNA COVID-19 shots and
- the results of a study that collected information on cardiac magnetic resonance imaging (cardiac MRI) in people who developed myocarditis after receiving an mRNA COVID-19 injection.
The FDA has also required the manufacturers to describe the new safety information in the adverse reactions section of the prescribing information and in the information for recipients and caregivers.
Additionally, the fact sheets for healthcare providers and for recipients and caregivers for Moderna COVID-19 shot and Pfizer-BioNTech COVID-19 shot, which are authorized for emergency use in individuals 6 months through 11 years of age, have also been updated to include the new safety information in alignment with the Comirnaty and Spikevax prescribing information and information for recipients and caregivers.
In a video published on social media, Dr. Vinay Prasad, director of the Center for Biologics Evaluation & Research Chief Medical and Scientific Officer, explained the alarming reasons for the warning updates.
While heart problems arose in approximately 8 out of 1 million persons ages 6 months to 64 years following reception of the cited shots, that number more than triples to 27 per million for males ages 12 to 24.
Prasad noted that multiple studies have arrived at similar findings.
Bruce Dowbiggin
The Covid 19 Disaster: When Do We Get The Apologies?

Breaking: Drs. Bonnie Henry and Theresa Tam have been appointed to the Order of Canada in recognition of their role in the country’s response to the COVID-19 pandemic.
And so the game of covid liar’s poker has more winners. It’s like awarding the captain of the Titanic the Nobel Prize for his work on floatation. As we now know these two— and the other WHO finger puppets in Canada— made the Covid 19 episode worse, not better, with their prescription for panic, positives and punishment. Even as they knew the truth about the limits of the virus and the efficacy of vaccines they continued to spew fallacious PCR data on the extent of the sickness and who was at risk.
Put simply, to protect vulnerable seniors they said kids were also at great risk. Which was unconscionable.
In this they encouraged Justin Trudeau in his worst instincts, combining his father’s insouciant disregard for civil rights (sending in the police) with his mother’s mental stability. Propped up by Team Tam and its U.S. allies such as Anthony Fauci, this hysteria peaked with a sequestered PM crushing the Truckers Convoy’s vaccine protest with emergency measures and destruction of civil liberties.
Lest you wonder, this overreach was recognized at the time. Justice Maclean wrote at the trial of Convoy organizers, “Defendants & other persons remain at liberty to engage in a peaceful, lawful & safe protest”. On Feb. 16, he continued a no-honking order, again writing: “Defendants & other persons remain at liberty to engage in a peaceful, lawful & safe protest.”
The leaders of the Convoy, lynched by Canadian media’s phoney claims of right-wing American interference, are still fighting jail time on charges of nuisance. While violent criminals are routinely released on bail or absolved.
Justice Richard Mosley later concluded that while the convoy was a disruption of public order, it didn’t constitute a national emergency and invoking the act “does not bear the hallmarks of reasonableness — justification, transparency and intelligibility.” But in real time Team Tam made no attempts to correct the wilder misgivings about Covid (lockdowns, mandatory vaccines). Trudeau was given a hall pass. Needless to say the purchased media made things infinitely worse regurgitating these mistakes.
In short, they knew better but hid the truth. But why pick on Henry and Tam? Under Trudeau and his wingman Jagmeet Singh this was the golden age of lies and prevarications in Canada and the U.S. No apologies were ever offered when the truth emerged.
As we’ve noted before, Trudeau cried with a teddy bear carefully positioned over 751 alleged unmarked graves in a known Catholic cemetery that the local Cowessess band abandoned. The Liberal government knew the claim of 215 “children’s graves” was false, and still ran with it to get Trudeau his photo-op. Naturally the CBC Media Party played (and still plays) accomplice in this farce as the Canadian flag was lowered to half-mast for six months and Trudeau ratted out Canada at the UN as a genocidal state.
There were more, plenty more Trudeau scandals that media endorsed and then stood by even as the truth was revealed. SNC Lavalin. We Charity. Arrive Can app. Firing indigenous justice minister. Chinese drug infiltration/ money laundering. Nazi Celebrated in Parliament. Welcome To Canada immigration. Nova Scotia massacre. McKinsey Consultation. Blackface. And so on.
And were there apologies when it came time to make the Trudeau Liberals accountable? No, they staged a media circus over Donald Trump’s assertion of 51st state. All the fake news and deliberate lies went poof, allowing Mark Carney to seamlessly assume the PM job.

Lest We Forget Pt. 2 it was not exclusive to Canada. As we are now learning: Barack Obama and Joe Biden sat in an August 3, 2016 Situation Room briefing and said, yeah, let the highest officials in our administration fabricate evidence to frame the opposing party candidate Donald Trump. Obama. Biden. Comey. McCabe. Strzok. Page. Rice. Etc.
Knowingly using the faked Clinton campaign ‘Steele Dossier’ hoax, they launched a federal investigation into the Trump presidential campaign that lasted three years after Trump was sworn in as the nation’s 45th President. Arresting and jailing his partners and colleagues. Inventing fake stories for their media enablers. Let’s repeat that. Saint Obama knew there was criminal activity in the process but let his henchmen try to fix an election.

And when the ruse was uncovered no one apologized. No one in authority was fired or jailed. The Pulitzer Prizes awarded to the NT Times and Washington Post for disseminating the DEMs scandal were not rescinded. Nor were they given back by the lying newspapers.
The concerted frauds of the same U.S. DOJ, FBI and State Departments were fed by media and accepted by gullible publics in Canada and America. The fantastical 2020 election results were likewise drummed into the public irrespective of the sudden “appearance” of 27 million new votes during a pandemic.
It was all a fitting preamble to the 2020-2024 Biden senility scandal with Democrats running a man they knew was in full dementia. In the 2020 election Biden was hidden from public view, the better to let media attack Trump for spurious charges launched by partisan DNC attorneys in Georgia, New York and DC. Even then it took the suppression of Hunter Biden’s incriminating laptop just prior to the election to get his father elected.

The dance of denial continued in Biden’s term as he physically and mentally deteriorated before the American public. But inquiries about who was running the government if not Biden were harshly suppressed. Media lackeys noted he was sharp as a tack mentally and in tip-top physical condition when he wasn’t falling down stairs.
It took the stunning 2024 debate debacle with Trump to strip away the lies about Biden’s health, now said to be advanced prostate cancer and Parkinson’s. The media, caught in their own lies about Biden’s condition, offered no apologies and tried to blame Biden’s stutter for the performance.. Right.
These were the two greatest U.S. hoaxes from people who’d cried hoax incessantly. They were hardly the only abuse of public trust. Some of the perpetrators are said to now be under investigation— even as they hand out awards to each other. The media’s credibility is shattered and yet they still blame others. Jaded voters are taking a “we’ll see” approach. But expectations of any change in DC or Ottawa are limited.
As Stephen Taylor posted on X: “Turns out for Liberals, ‘elbows up’ just means ‘noses up’ like it always has.”
Bruce Dowbiggin @dowbboy is the editor of Not The Public Broadcaster A two-time winner of the Gemini Award as Canada’s top television sports broadcaster, his new book Deal With It: The Trades That Stunned The NHL And Changed hockey is now available on Amazon. Inexact Science: The Six Most Compelling Draft Years In NHL History, his previous book with his son Evan, was voted the seventh-best professional hockey book of all time by bookauthority.org . His 2004 book Money Players was voted sixth best on the same list, and is available via brucedowbigginbooks.ca.
-

 COVID-1916 hours ago
COVID-1916 hours agoFDA requires new warning on mRNA COVID shots due to heart damage in young men
-

 Business14 hours ago
Business14 hours agoCarney’s new agenda faces old Canadian problems
-

 Daily Caller11 hours ago
Daily Caller11 hours agoBlackouts Coming If America Continues With Biden-Era Green Frenzy, Trump Admin Warns
-

 Indigenous15 hours ago
Indigenous15 hours agoInternal emails show Canadian gov’t doubted ‘mass graves’ narrative but went along with it
-

 Bruce Dowbiggin17 hours ago
Bruce Dowbiggin17 hours agoEau Canada! Join Us In An Inclusive New National Anthem
-

 Business2 days ago
Business2 days agoUN’s ‘Plastics Treaty’ Sports A Junk Science Wrapper
-

 Environment2 days ago
Environment2 days agoEPA releases report on chemtrails, climate manipulation
-

 Business2 days ago
Business2 days agoCBC six-figure salaries soar


