COVID-19
71% of reported adverse reactions to COVID-19 vaccine from just 4.2% of vaccine batches: Danish study
From Dr. John Campbell on YouTube
A report “Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine” by Danish researchers Max Schmeling, Vibeke Manniche, Peter Riis Hansen is shedding light on the number of adverse reactions to specific batches of the Pfizer-BioNTech’s COVID-19 vaccines.
The researchers note in their report
“Vaccine vials with individual doses are supplied in batches with stringent quality control to ensure batch and dose uniformity.2 Clinical data on individual vaccine batch levels have not been reported and batch-dependent variation in the clinical efficacy and safety of authorized vaccines would appear to be highly unlikely. However, not least in view of the emergency use market authorization and rapid implementation of large-scale vaccination programs, the possibility of batch-dependent variation appears worthy of investigation. We therefore examined rates of SAEs between different BNT162b2 vaccine batches administered in Denmark (population 5.8 million) from 27 December 2020 to 11 January 2022.”
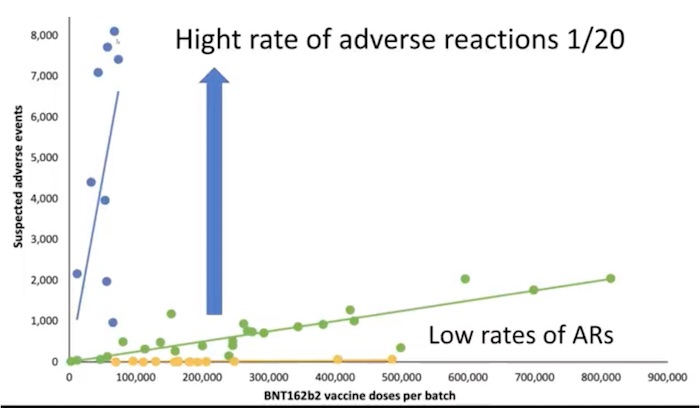
The results of the study are astounding. In certain vaccine batches reported adverse reactions occurred in one out of every twenty shots. Other batches have yet to be associated with any adverse reactions at all. As British health researcher Dr. John Campbell explains, this study could pose very serious questions Pfizer must address.
Viral Vaccine paper
Dr. Campbell’s presentation notes:
Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine
https://onlinelibrary.wiley.com/doi/1…
71% of the suspected adverse reactions occurred in 4.2% of the vaccine batches
Numbers of suspected adverse events (SAEs), after BNT612b2 mRNA vaccination in Denmark.
27 December 2020–11 January 2022, (population 5.8 million)
(According to the number of doses per vaccine batch)
Each dot represents a single vaccine batch.
By 11 November 2022 (European area) 701 million doses of Pfizer given 971,021 reports of suspected adverse effects (SAEs)
Clinical data on individual vaccine batch levels have not been reported (batch-dependent variation in the clinical efficacy and safety of authorized vaccines would appear to be highly unlikely)
We therefore examined rates of SAEs between different BNT162b2 vaccine batches administered in Denmark
Data on all SAE cases, Danish Medical Agency (DKMA) SAE seriousness was classified as non-serious, serious (hospitalization or prolongation of existing hospitalization, life-threatening illness, permanent disability or congenital malformation) or SAE-related d****
Anonymized data SAEs were counted on a batch level by linking individual SAEs to the batch label(s) of BNT162b dose(s)
10,793,766 doses administered 4,026,575 persons 52 different BNT162b2 vaccine batches (2,340–814,320 doses per batch)
43,496 SAEs were registered in 13,635 persons 61,847 batch-identifiable SAEs, of which 14,509 (23.5%) were classified as severe, 579 (0.9%) were SAE-related d*****
Unexpectedly Rates of SAEs per 1000 doses varied considerably between vaccine batches
From 1 SAE per 20 doses given to I in many thousands to zero
Variabilities
Vaccine manufacturing
Storage
Transportation
Clinical handling and control
Administration technique
Viral vaccine paper, Dr Vibeke Manniche
COVID-19
Trump DOJ dismisses charges against doctor who issued fake COVID passports

From LifeSiteNews
Attorney General Pam Bondi has ended the federal prosecution of Dr. Michael Kirk Moore for giving ‘patients a choice when the federal government refused to do so.’
The Utah plastic surgeon who issued fake COVID-19 vaccine passports to help patients get around COVID vaccine mandates will no longer be prosecuted, U.S. Attorney General Pam Bondi announced Saturday.
During the COVID pandemic, Dr. Michael Kirk Moore Jr. and employees at his Salt Lake private practice developed a plan to provide patients who objected to being forced to take the vaccine with ineffectual, harmless saline injections instead and give them COVID vaccination cards that would satisfy (since rescinded) mandates to take the shot as a condition of employment, public facilities, mass gatherings, and more.
For his efforts, he was indicted for allegedly “endanger[ing] the health and well-being of a vulnerable population” and “undermin[ing] public trust and the integrity of federal health care programs.” The government also accused him of doing so for profit, but several sources attested off the record that Moore not only issued the cards for free but actually refused offers of compensation.
“They broke no laws and harmed no person,” the defendants’ legal team said in 2023. “Dr. Moore, specifically, abided by his long held Hippocratic oath to First Do No Harm. We believe he and his co-defendants will be found innocent of all charges.”
Last month, LifeSiteNews reported that Moore’s trial was set to begin on July 7, which could have potentially ended with him facing 35 years in jail and a $125,000 penalty. Supporters of the doctor had expressed worry that the change in presidential administration had not yet halted the prosecution.
Over the weekend, however, Bondi announced that at her direction it has now done exactly that.
“Dr. Moore gave his patients a choice when the federal government refused to do so,” she said. “He did not deserve the years in prison he was facing. It ends today.”
There is a large body of warning signs against the shots, which were developed in record time by the first Trump administration’s Operation Warp Speed initiative.
The federal Vaccine Adverse Event Reporting System (VAERS) reports 38,709 deaths, 221,030 hospitalizations, 22,331 heart attacks, and 28,966 myocarditis and pericarditis cases as of June 27, among other ailments. U.S. Centers for Disease Control & Prevention (CDC) researchers have recognized a “high verification rate of reports of myocarditis to VAERS after mRNA-based COVID-19 vaccination,” leading to the conclusion that “under-reporting is more likely” than over-reporting.
An analysis of 99 million people across eight countries published in the journal Vaccine “observed significantly higher risks of myocarditis following the first, second and third doses” of mRNA-based COVID vaccines, as well as signs of increased risk of “pericarditis, Guillain-Barré syndrome, and cerebral venous sinus thrombosis,” and other “potential safety signals that require further investigation.”
In April 2024, the U.S. Centers for Disease Control & Prevention (CDC) was forced to release by court order 780,000 previously undisclosed reports of serious adverse reactions, and a study out of Japan found “statistically significant increases” in cancer deaths after third doses of mRNA-based COVID-19 vaccines, and offered several theories for a causal link.
In January, a long-awaited Florida grand jury report on the COVID vaccine manufacturers found that while only a miniscule percentage of the millions of vaccinations resulted in serious harm based on the data it had access to, such events do occur, and there are “profound and serious issues” in pharmaceutical companies’ review process, including reluctance to share what evidence of adverse events they did find.
In May, Trump administration U.S. Food & Drug Administration (FDA) Commissioner Dr. Marty Makary and vaccine chief Dr. Vinay Prasad announced that there would no longer be blanket recommendations for all Americans to receive the shot, but the “risk factors” it would still be recommended for include asthma, cancer, cerebrovascular disease, chronic kidney diseases, a handful of chronic liver and lung diseases, diabetes, disabilities such as Down’s syndrome, heart conditions, HIV, dementia, Parkinson’s, obesity, smoking, tuberculosis, and more. Health & Human Services (HHS) Secretary Robert F. Kennedy Jr. subsequently announced COVID vaccines will not be recommended to healthy children or pregnant women.
The Trump administration has approved a new mRNA-based COVID-19 vaccine from Moderna, suggesting the federal government’s overall view of the shots will remain favorable, albeit without mandates of any kind. At the same time, it does require mRNA COVID shots to carry a new warning about the danger of heart damage in young men.
Business
Carney Liberals quietly award Pfizer, Moderna nearly $400 million for new COVID shot contracts

From LifeSiteNews
Carney’s Liberal government signed nearly $400 million in contracts with Pfizer and Moderna for COVID shots, despite halted booster programs and ongoing delays in compensating Canadians for jab injuries.
Prime Minister Mark Carney has awarded Pfizer and Moderna nearly $400 million in new COVID shot contracts.
On June 30th, the Liberal government quietly signed nearly $400 million contracts with vaccine companies Pfizer and Moderna for COVID jabs, despite thousands of Canadians waiting to receive compensation for COVID shot injuries.
The contracts, published on the Government of Canada website, run from June 30, 2025, until March 31, 2026. Under the contracts, taxpayers must pay $199,907,418.00 to both companies for their COVID shots.
Notably, there have been no press releases regarding the contracts on the Government of Canada website nor from Carney’s official office.
Additionally, the contracts were signed after most Canadians provinces halted their COVID booster shot programs. At the same time, many Canadians are still waiting to receive compensation from COVID shot injuries.
Canada’s Vaccine Injury Support Program (VISP) was launched in December 2020 after the Canadian government gave vaccine makers a shield from liability regarding COVID-19 jab-related injuries.
There has been a total of 3,317 claims received, of which only 234 have received payments. In December, the Canadian Department of Health warned that COVID shot injury payouts will exceed the $75 million budget.
The December memo is the last public update that Canadians have received regarding the cost of the program. However, private investigations have revealed that much of the funding is going in the pockets of administrators, not injured Canadians.
A July report by Global News discovered that Oxaro Inc., the consulting company overseeing the VISP, has received $50.6 million. Of that fund, $33.7 million has been spent on administrative costs, compared to only $16.9 million going to vaccine injured Canadians.
Furthermore, the claims do not represent the total number of Canadians injured by the allegedly “safe and effective” COVID shots, as inside memos have revealed that the Public Health Agency of Canada (PHAC) officials neglected to report all adverse effects from COVID jabs and even went as far as telling staff not to report all events.
The PHAC’s downplaying of jab injuries is of little surprise to Canadians, as a 2023 secret memo revealed that the federal government purposefully hid adverse effect so as not to alarm Canadians.
The secret memo from former Prime Minister Justin Trudeau’s Privy Council Office noted that COVID jab injuries and even deaths “have the potential to shake public confidence.”
“Adverse effects following immunization, news reports and the government’s response to them have the potential to shake public confidence in the COVID-19 vaccination rollout,” read a part of the memo titled “Testing Behaviourally Informed Messaging in Response to Severe Adverse Events Following Immunization.”
Instead of alerting the public, the secret memo suggested developing “winning communication strategies” to ensure the public did not lose confidence in the experimental injections.
Since the start of the COVID crisis, official data shows that the virus has been listed as the cause of death for less than 20 children in Canada under age 15. This is out of six million children in the age group.
The COVID jabs approved in Canada have also been associated with severe side effects, such as blood clots, rashes, miscarriages, and even heart attacks in young, healthy men.
Additionally, a recent study done by researchers with Canada-based Correlation Research in the Public Interest showed that 17 countries have found a “definite causal link” between peaks in all-cause mortality and the fast rollouts of the COVID shots, as well as boosters.
Interestingly, while the Department of Health has spent $16 million on injury payouts, the Liberal government spent $54 million COVID propaganda promoting the shot to young Canadians.
The Public Health Agency of Canada especially targeted young Canadians ages 18-24 because they “may play down the seriousness of the situation.”
-

 Fraser Institute1 day ago
Fraser Institute1 day agoBefore Trudeau average annual immigration was 617,800. Under Trudeau number skyrocketted to 1.4 million annually
-

 MAiD1 day ago
MAiD1 day agoCanada’s euthanasia regime is already killing the disabled. It’s about to get worse
-

 Frontier Centre for Public Policy1 day ago
Frontier Centre for Public Policy1 day agoNew Book Warns The Decline In Marriage Comes At A High Cost
-

 Business1 day ago
Business1 day agoPrime minister can make good on campaign promise by reforming Canada Health Act
-

 Addictions1 day ago
Addictions1 day ago‘Over and over until they die’: Drug crisis pushes first responders to the brink
-

 International1 day ago
International1 day agoChicago suburb purchases childhood home of Pope Leo XIV
-

 Daily Caller1 day ago
Daily Caller1 day agoUSAID Quietly Sent Thousands Of Viruses To Chinese Military-Linked Biolab
-

 Business2 days ago
Business2 days ago103 Conflicts and Counting Unprecedented Ethics Web of Prime Minister Mark Carney