Health
Pharmacare won’t help Canadians with rare disorders
From the MacDonald Laurier Institute
By Nigel Rawson and John Adams
Canadians with rare disorders will be even worse off if NDP’s parliamentary blackmail works
Last month the federal NDP convention in Hamilton voted unanimously to force the Liberals to introduce a single-payer universal pharmacare program or see the current “confidence-and-supply” deal canceled. Will universal government-run pharmacare benefit Canadians with rare disorders? We fear not.
Canadians with such disorders are already disadvantaged compared with sufferers in other countries. Fewer specialized drugs are launched in Canada than in the United States and Europe. Those that are get approval for marketing about a year, on average, after they do there.
That’s not because Health Canada takes longer to review new medicines. The process takes about the same time in the three places. Rather, delayed approval is likely due to manufacturers submitting later to Health Canada because federal, provincial and territorial hostility towards the industry has made our biopharmaceutical market less attractive.
Approval doesn’t mean government drug plans will pay for a drug, however. Further government-created barriers impact all Canadians, but particularly those with rare disorders who want access to novel drugs for their unmet or poorly met health needs. As a consequence, what gets listed in government drug plans varies widely, leading to a postal code lottery.
In a set of articles published over the summer by the Macdonald-Laurier Institute, we discuss the several obstacles patients and their families face as they try to gain access to new or expensive innovative therapies. They include: the lack of federal incentives for developers to submit new medicines to Health Canada; health technology assessment that is neither accountable, independent nor transparent and makes recommendations about which drugs to cover in public drug plans to governments; and price negotiations between government drug plans and manufacturers.
Even when drug developers clear these government-created barriers, public drug plans are under no obligation to add the approved medicines to their benefit lists. Too often governments focus only on drug costs and ignore the broader benefits effective drugs can bring, not only to the health and well-being of patients and their families, but also to other parts of the health system, to the economy and to society at large. If a new drug reduces doctor or emergency visits or hospitalizations or helps a person get back to work, those benefits typically are ignored by our drug assessment system.
The federal government made matters worse over the past six years by planning to drastically reduce drug prices by regulatory order, not negotiation. This caused considerable uncertainty among developers, resulting in even fewer new drugs being submitted for marketing approval here than in the U.S. and EU.
Proponents plainly want a lowest-common-denominator government-run public plan that would crowd out private plans, which over two-thirds of Canadians currently rely on for drug access.
Despite the federal government committing $1.5 billion over three years to “increase access to, and affordability of, effective drugs for rare diseases to improve the health of patients across Canada,” its initiative is not comprehensive. So far, Canada has neither a government-endorsed national rare disorder strategy nor an Orphan Drug Act providing incentives to developers to launch orphan medicines in Canada. Most other developed countries have both.
Patients’ organizations have stepped in where governments have failed to act and proposed a Canadian strategy that would include incentives and funding to encourage developers to launch drugs in this country and cut through the barriers we have described to provide timely access to the many innovative treatments on the research horizon. For example, access to breakthrough drugs could be allowed as soon as Health Canada says they are safe and effective, even as other administrative boxes are checked and prices negotiated. Other countries use this approach.
Canadians afflicted with any of the 11,000 or so known rare disorders have significant unmet needs. Fewer than five per cent have any treatment beyond symptom relief or palliative care. The last thing these people need is for governments to ration innovative drugs even more than they already do or to force even deeper price cuts from drug developers in order to pay for universal pharmacare that covers only basic medicines.
Canadians with rare disorders almost certainly will be even worse off if the NDP’s parliamentary blackmail works.
Nigel Rawson is an affiliate scholar with the Canadian Health Policy Institute and a senior fellow with the Macdonald-Laurier Institute, as is John Adams, co-founder and CEO of Canadian PKU and Allied Disorders Inc.
Alberta
A Christmas wish list for health-care reform

From the Fraser Institute
By Nadeem Esmail and Mackenzie Moir
It’s an exciting time in Canadian health-care policy. But even the slew of new reforms in Alberta only go part of the way to using all the policy tools employed by high performing universal health-care systems.
For 2026, for the sake of Canadian patients, let’s hope Alberta stays the path on changes to how hospitals are paid and allowing some private purchases of health care, and that other provinces start to catch up.
While Alberta’s new reforms were welcome news this year, it’s clear Canada’s health-care system continued to struggle. Canadians were reminded by our annual comparison of health care systems that they pay for one of the developed world’s most expensive universal health-care systems, yet have some of the fewest physicians and hospital beds, while waiting in some of the longest queues.
And speaking of queues, wait times across Canada for non-emergency care reached the second-highest level ever measured at 28.6 weeks from general practitioner referral to actual treatment. That’s more than triple the wait of the early 1990s despite decades of government promises and spending commitments. Other work found that at least 23,746 patients died while waiting for care, and nearly 1.3 million Canadians left our overcrowded emergency rooms without being treated.
At least one province has shown a genuine willingness to do something about these problems.
The Smith government in Alberta announced early in the year that it would move towards paying hospitals per-patient treated as opposed to a fixed annual budget, a policy approach that Quebec has been working on for years. Albertans will also soon be able purchase, at least in a limited way, some diagnostic and surgical services for themselves, which is again already possible in Quebec. Alberta has also gone a step further by allowing physicians to work in both public and private settings.
While controversial in Canada, these approaches simply mirror what is being done in all of the developed world’s top-performing universal health-care systems. Australia, the Netherlands, Germany and Switzerland all pay their hospitals per patient treated, and allow patients the opportunity to purchase care privately if they wish. They all also have better and faster universally accessible health care than Canada’s provinces provide, while spending a little more (Switzerland) or less (Australia, Germany, the Netherlands) than we do.
While these reforms are clearly a step in the right direction, there’s more to be done.
Even if we include Alberta’s reforms, these countries still do some very important things differently.
Critically, all of these countries expect patients to pay a small amount for their universally accessible services. The reasoning is straightforward: we all spend our own money more carefully than we spend someone else’s, and patients will make more informed decisions about when and where it’s best to access the health-care system when they have to pay a little out of pocket.
The evidence around this policy is clear—with appropriate safeguards to protect the very ill and exemptions for lower-income and other vulnerable populations, the demand for outpatient healthcare services falls, reducing delays and freeing up resources for others.
Charging patients even small amounts for care would of course violate the Canada Health Act, but it would also emulate the approach of 100 per cent of the developed world’s top-performing health-care systems. In this case, violating outdated federal policy means better universal health care for Canadians.
These top-performing countries also see the private sector and innovative entrepreneurs as partners in delivering universal health care. A relationship that is far different from the limited individual contracts some provinces have with private clinics and surgical centres to provide care in Canada. In these other countries, even full-service hospitals are operated by private providers. Importantly, partnering with innovative private providers, even hospitals, to deliver universal health care does not violate the Canada Health Act.
So, while Alberta has made strides this past year moving towards the well-established higher performance policy approach followed elsewhere, the Smith government remains at least a couple steps short of truly adopting a more Australian or European approach for health care. And other provinces have yet to even get to where Alberta will soon be.
Let’s hope in 2026 that Alberta keeps moving towards a truly world class universal health-care experience for patients, and that the other provinces catch up.
Health
FDA warns ‘breast binder’ manufacturers to stop marketing to gender-confused girls

From LifeSiteNews
Dr. Marty Makary took aim at the transgender-medical-industrial complex that has exploded in recent years during a recent press conference.
Food and Drug Administration (FDA) commissioner Dr. Marty Makary has sternly warned companies manufacturing “breast binders” to cease marketing and supplying their product to gender-confused girls seeking to make their bodies appear masculine.
“Today the FDA is taking action,” said Makary in a press conference. “We are sending warning letters to 12 manufacturers and retailers for illegal marketing of breast binders for children, for the purposes of treating gender dysphoria.”
“Breast binders are a class one medical device with legitimate medical users, such as being used by women after breast cancer surgery,” but “these binders are not benign,” he cautioned. “Long-term usage has been associated with pain, compromised lung function, and even difficulty breast feeding later in life.”
“The warning letters will formally notify the companies of their significant regulatory violations and require prompt corrective action,” said the FDA head.
.@DrMakaryFDA: “Today the FDA is taking action. We are sending warning letters to 12 manufacturers and retailers for illegal marketing of breast binders for children, for the purposes of treating gender dysphoria.” pic.twitter.com/6JNAy36223
— HHS Rapid Response (@HHSResponse) December 18, 2025
The warning letter addressed to California manufacturer, GenderBender, notes that the company’s website states that “[c]hest binding is the practice of compressing breast mass into a more masculine shape, often done in the LGBTQ community for gender euphoria.”
“Your firm should take prompt action to address any violations identified in this letter. Failure to adequately address this matter may result in regulatory action being initiated by the FDA without further notice. These actions include, but are not limited to, seizure and injunction,” advised the FDA.
During his presentation, Makary took aim at the transgender-medical-industrial complex that has exploded in recent years.
“One of the most barbaric features of a society is the genital mutilation of its children,” observed Makary.
“Pushing transgender ideology in children is predatory, it’s wrong, and it needs to stop,” he declared.
“This ideology is a belief system that some teachers, some pediatricians, and others are selling to children without their parents knowing sometimes, or with a deliberate attempt to remove parents from the decision making,” Makary explained.
To witness society “putting kids on a path of chest binders, drugs, castration, mastectomies, and other procedures is a path that now many kids regret,” he lamented, as he pointed to Chloe Cole, who has reverted to her God-given femininity after undergoing so-called “gender-affirming” surgery as a teen.
Cole is a leading voice for young people who have “detransitioned” after having medically, surgically, and socially attempted to “transition” to a member of the opposite sex.
.@DrMakaryFDA: “Pushing transgender ideology in children is predatory, it's wrong, and it needs to stop.” pic.twitter.com/TXxWNEtNZk
— HHS Rapid Response (@HHSResponse) December 18, 2025
-

 Haultain Research2 days ago
Haultain Research2 days agoSweden Fixed What Canada Won’t Even Name
-

 Business2 days ago
Business2 days agoWhat Do Loyalty Rewards Programs Cost Us?
-

 Business1 day ago
Business1 day agoLand use will be British Columbia’s biggest issue in 2026
-

 Energy19 hours ago
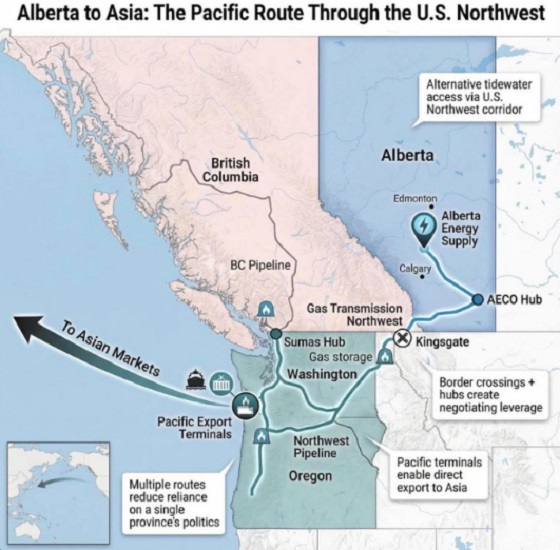
Energy19 hours agoRulings could affect energy prices everywhere: Climate activists v. the energy industry in 2026
-

 International18 hours ago
International18 hours agoChina Stages Massive Live-Fire Encirclement Drill Around Taiwan as Washington and Japan Fortify
-

 Digital ID17 hours ago
Digital ID17 hours agoThe Global Push for Government Mandated Digital IDs And Why You Should Worry
-

 Business15 hours ago
Business15 hours agoFeds pull the plug on small business grants to Minnesota after massive fraud reports
-

 Business4 hours ago
Business4 hours agoDOOR TO DOOR: Feds descend on Minneapolis day cares tied to massive fraud