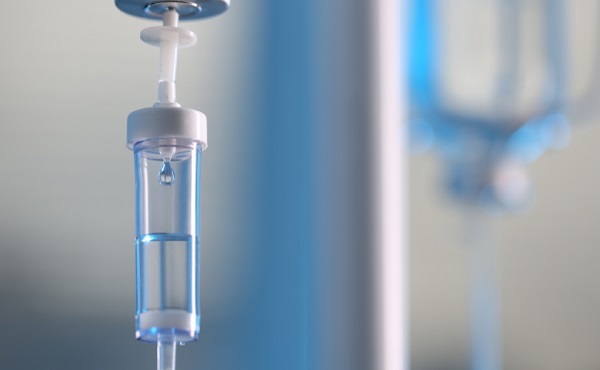
Health
Quadriplegic man dies via euthanasia after developing bed sores waiting at Quebec hospital

66-year-old Quebec man Normand Meunier who died via euthanasia after a 4-day hospital stay left him with severe bed sores
From LifeSiteNews
‘I don’t want to be a burden,’ the 66-year-old man said prior to his death after he developed bed sores due to a lack of specialized care at a hospital in Saint-Jérôme, Quebec.
A quadriplegic man in Quebec was killed via euthanasia after he developed severe bed sores while waiting in a hospital for an extended period of time.
On March 29, Normand Meunier, a 66-year-old quadriplegic man in Quebec, was euthanized in his home after developing bed sores due to a lack of specialized care at the hospital in Saint-Jérôme, Quebec, according to a report by Radio-Canada.
“I don’t want to be a burden. At any rate, the medical opinions say I won’t be a burden for long; as the old folks say, it’s better to kick the can,” Meunier told Radio-Canada in an interview the day before he was killed.
Meunier, whose arms and legs have been paralyzed since 2022 due to a spinal cord injury, went to the hospital’s intensive care for a respiratory virus. According to his partner Sylvie Brosseau, the hospital placed Meunier on a stretcher for 95 hours.
Bosseau revealed that she asked medical staff to provide a specialized bed for Meunier but was told that the hospital would have to order one. According to the hospital, they are investigating the incident, adding that they do have beds available.
After spending four days on a hospital cot, Meunier developed bed sores and a major pressure ulcer on his buttocks, which were so severe that the muscle and bone were exposed and visible.
While Meunier had previously experienced bedsores, he determined to end his life via Medical Assistance in Dying (MAiD), the euphemistic name for Canada’s euthanasia regime, rather than continue to receive treatment.
Unfortunately, Meunier is not the first Canadians to choose MAiD after being given insufficient medical care.
As a result of a healthcare worker shortage, wait times to receive care in Canada have increased to an average of 27.7 weeks, leading some Canadians to despair and opt for euthanasia instead of waiting for assistance.
This was the case of 52-year-old Dan Quayle, a grandfather from British Columbia. On November 24, he chose to be killed via lethal injection after being unable to receive cancer treatment due to the increased wait times.
Throughout the agonizing wait, his family “prayed he would change his mind or get an 11th-hour call that chemo had been scheduled,” but were instead told consistently by the hospital that they were “backlogged.”
Similarly, in 2022, a Winnipeg woman wrote in her posthumously published obituary that she chose to die by assisted suicide after being refused the treatments she needed: “I could have had more time if I had more help.”
However, instead of supporting the healthcare system to prevent Canadians from taking their own lives, the Trudeau government is working to expand access to MAiD by loosening its requirements.
On March 9, 2024, MAiD was set to expand to include those suffering solely from mental illness. This is a result of the 2021 passage of Bill C-7, which also allowed the chronically ill – not just the terminally ill – to qualify for so-called doctor-assisted death.
After massive pushback from doctors, pro-life groups and politicians, the program’s expansion was temporarily paused until 2027.
According to Health Canada, in 2022, 13,241 Canadians died by MAiD lethal injection, which is 4.1 percent of all deaths in the country for that year, and a 31.2 percent increase from 2021.
The number of Canadians killed by lethal injection since 2016 now stands at 44,958.
Brownstone Institute
FDA Exposed: Hundreds of Drugs Approved without Proof They Work

From the Brownstone Institute
By
The US Food and Drug Administration (FDA) has approved hundreds of drugs without proof that they work—and in some cases, despite evidence that they cause harm.
That’s the finding of a blistering two-year investigation by medical journalists Jeanne Lenzer and Shannon Brownlee, published by The Lever.
Reviewing more than 400 drug approvals between 2013 and 2022, the authors found the agency repeatedly ignored its own scientific standards.
One expert put it bluntly—the FDA’s threshold for evidence “can’t go any lower because it’s already in the dirt.”
A System Built on Weak Evidence
The findings were damning—73% of drugs approved by the FDA during the study period failed to meet all four basic criteria for demonstrating “substantial evidence” of effectiveness.
Those four criteria—presence of a control group, replication in two well-conducted trials, blinding of participants and investigators, and the use of clinical endpoints like symptom relief or extended survival—are supposed to be the bedrock of drug evaluation.
Yet only 28% of drugs met all four criteria—40 drugs met none.
These aren’t obscure technicalities—they are the most basic safeguards to protect patients from ineffective or dangerous treatments.
But under political and industry pressure, the FDA has increasingly abandoned them in favour of speed and so-called “regulatory flexibility.”
Since the early 1990s, the agency has relied heavily on expedited pathways that fast-track drugs to market.
In theory, this balances urgency with scientific rigour. In practice, it has flipped the process. Companies can now get drugs approved before proving that they work, with the promise of follow-up trials later.
But, as Lenzer and Brownlee revealed, “Nearly half of the required follow-up studies are never completed—and those that are often fail to show the drugs work, even while they remain on the market.”
“This represents a seismic shift in FDA regulation that has been quietly accomplished with virtually no awareness by doctors or the public,” they added.
More than half the approvals examined relied on preliminary data—not solid evidence that patients lived longer, felt better, or functioned more effectively.
And even when follow-up studies are conducted, many rely on the same flawed surrogate measures rather than hard clinical outcomes.
The result: a regulatory system where the FDA no longer acts as a gatekeeper—but as a passive observer.
Cancer Drugs: High Stakes, Low Standards
Nowhere is this failure more visible than in oncology.
Only 3 out of 123 cancer drugs approved between 2013 and 2022 met all four of the FDA’s basic scientific standards.
Most—81%—were approved based on surrogate endpoints like tumour shrinkage, without any evidence that they improved survival or quality of life.
Take Copiktra, for example—a drug approved in 2018 for blood cancers. The FDA gave it the green light based on improved “progression-free survival,” a measure of how long a tumour stays stable.
But a review of post-marketing data showed that patients taking Copiktra died 11 months earlier than those on a comparator drug.
It took six years after those studies showed the drug reduced patients’ survival for the FDA to warn the public that Copiktra should not be used as a first- or second-line treatment for certain types of leukaemia and lymphoma, citing “an increased risk of treatment-related mortality.”
Elmiron: Ineffective, Dangerous—And Still on the Market
Another striking case is Elmiron, approved in 1996 for interstitial cystitis—a painful bladder condition.
The FDA authorized it based on “close to zero data,” on the condition that the company conduct a follow-up study to determine whether it actually worked.
That study wasn’t completed for 18 years—and when it was, it showed Elmiron was no better than placebo.
In the meantime, hundreds of patients suffered vision loss or blindness. Others were hospitalized with colitis. Some died.
Yet Elmiron is still on the market today. Doctors continue to prescribe it.
“Hundreds of thousands of patients have been exposed to the drug, and the American Urological Association lists it as the only FDA-approved medication for interstitial cystitis,” Lenzer and Brownlee reported.
“Dangling Approvals” and Regulatory Paralysis
The FDA even has a term—”dangling approvals”—for drugs that remain on the market despite failed or missing follow-up trials.
One notorious case is Avastin, approved in 2008 for metastatic breast cancer.
It was fast-tracked, again, based on ‘progression-free survival.’ But after five clinical trials showed no improvement in overall survival—and raised serious safety concerns—the FDA moved to revoke its approval for metastatic breast cancer.
The backlash was intense.
Drug companies and patient advocacy groups launched a campaign to keep Avastin on the market. FDA staff received violent threats. Police were posted outside the agency’s building.
The fallout was so severe that for more than two decades afterwards, the FDA did not initiate another involuntary drug withdrawal in the face of industry opposition.
Billions Wasted, Thousands Harmed
Between 2018 and 2021, US taxpayers—through Medicare and Medicaid—paid $18 billion for drugs approved under the condition that follow-up studies would be conducted. Many never were.
The cost in lives is even higher.
A 2015 study found that 86% of cancer drugs approved between 2008 and 2012 based on surrogate outcomes showed no evidence that they helped patients live longer.
An estimated 128,000 Americans die each year from the effects of properly prescribed medications—excluding opioid overdoses. That’s more than all deaths from illegal drugs combined.
A 2024 analysis by Danish physician Peter Gøtzsche found that adverse effects from prescription medicines now rank among the top three causes of death globally.
Doctors Misled by the Drug Labels
Despite the scale of the problem, most patients—and most doctors—have no idea.
A 2016 survey published in JAMA asked practising physicians a simple question—what does FDA approval actually mean?
Only 6% got it right.
The rest assumed that it meant the drug had shown clear, clinically meaningful benefits—such as helping patients live longer or feel better—and that the data was statistically sound.
But the FDA requires none of that.
Drugs can be approved based on a single small study, a surrogate endpoint, or marginal statistical findings. Labels are often based on limited data, yet many doctors take them at face value.
Harvard researcher Aaron Kesselheim, who led the survey, said the results were “disappointing, but not entirely surprising,” noting that few doctors are taught about how the FDA’s regulatory process actually works.
Instead, physicians often rely on labels, marketing, or assumptions—believing that if the FDA has authorized a drug, it must be both safe and effective.
But as The Lever investigation shows, that is not a safe assumption.
And without that knowledge, even well-meaning physicians may prescribe drugs that do little good—and cause real harm.
Who Is the FDA Working for?
In interviews with more than 100 experts, patients, and former regulators, Lenzer and Brownlee found widespread concern that the FDA has lost its way.
Many pointed to the agency’s dependence on industry money. A BMJ investigation in 2022 found that user fees now fund two-thirds of the FDA’s drug review budget—raising serious questions about independence.

Yale physician and regulatory expert Reshma Ramachandran said the system is in urgent need of reform.
“We need an agency that’s independent from the industry it regulates and that uses high-quality science to assess the safety and efficacy of new drugs,” she told The Lever. “Without that, we might as well go back to the days of snake oil and patent medicines.”
For now, patients remain unwitting participants in a vast, unspoken experiment—taking drugs that may never have been properly tested, trusting a regulator that too often fails to protect them.
And as Lenzer and Brownlee conclude, that trust is increasingly misplaced.
- Investigative report by Jeanne Lenzer and Shannon Brownlee at The Lever [link]
- Searchable public drug approval database [link]
- See my talk: Failure of Drug Regulation: Declining standards and institutional corruption
Republished from the author’s Substack
Health
Red Deer Hospital Lottery 2025 Winners

The Red Deer Regional Health Foundation is thrilled to announce the winners of this year’s Red Deer Hospital Lottery prizes – including the Dream Home, a $100,000.00 cash prize, and Mega Bucks 50.
James Smith of Spruce View has won the $100,000.00 cash prize.
Montey Brehaut of Red Deer has won the Mega Bucks 50 jackpot, taking home $301,702.50.
The grand prize Sorento Custom Homes Dream Home, including furnishings by Urban Barn and worth $1,074,472 – has been awarded to Oscar Gunnlaugson of Sylvan Lake.
The winner announcements took place at noon on June 26 , 2025 – and was streamed live on Facebook from Red Deer Regional Hospital Center.
“We’re excited to celebrate this year’s winners and deeply grateful to everyone who supported the lottery,” said Manon Therriault, CEO of the Red Deer Regional Health Foundation. “Funds raised will directly enhance patient care at Red Deer Regional Hospital Centre.”
This year’s lottery proceeds will fund essential new and replacement equipment, ensuring Red Deer Regional Hospital Center can continue to serve the 500,000 people who rely on it. While plans for the hospital expansion move forward, healthcare doesn’t wait. Patients in our community need access
to life-saving technology today, and supporting Red Deer Hospital Lottery has made that possible.
A full list of winners, including electronics prize recipients, will be posted on July 2 at reddeerhospitallottery.ca.
Winners will also receive instructions on how to claim their prizes by mail.
The keys to the Dream Home will be presented at a special ceremony this summer.
-

 Alberta6 hours ago
Alberta6 hours agoAlberta Independence Seekers Take First Step: Citizen Initiative Application Approved, Notice of Initiative Petition Issued
-

 Automotive1 day ago
Automotive1 day agoElectric vehicle sales are falling hard in BC, and it is time to recognize reality.
-

 Automotive1 day ago
Automotive1 day agoPower Struggle: Electric vehicles and reality
-
 Business1 day ago
Business1 day agoTrump on Canada tariff deadline: ‘We can do whatever we want’
-

 Business9 hours ago
Business9 hours agoCanada Caves: Carney ditches digital services tax after criticism from Trump
-

 Crime9 hours ago
Crime9 hours agoSuspected ambush leaves two firefighters dead in Idaho
-

 Brownstone Institute2 days ago
Brownstone Institute2 days agoFDA Exposed: Hundreds of Drugs Approved without Proof They Work
-

 Energy1 day ago
Energy1 day agoChina undermining American energy independence, report says